How a Sample Inventory Management System Supports Scaling from Phase I to Commercialization
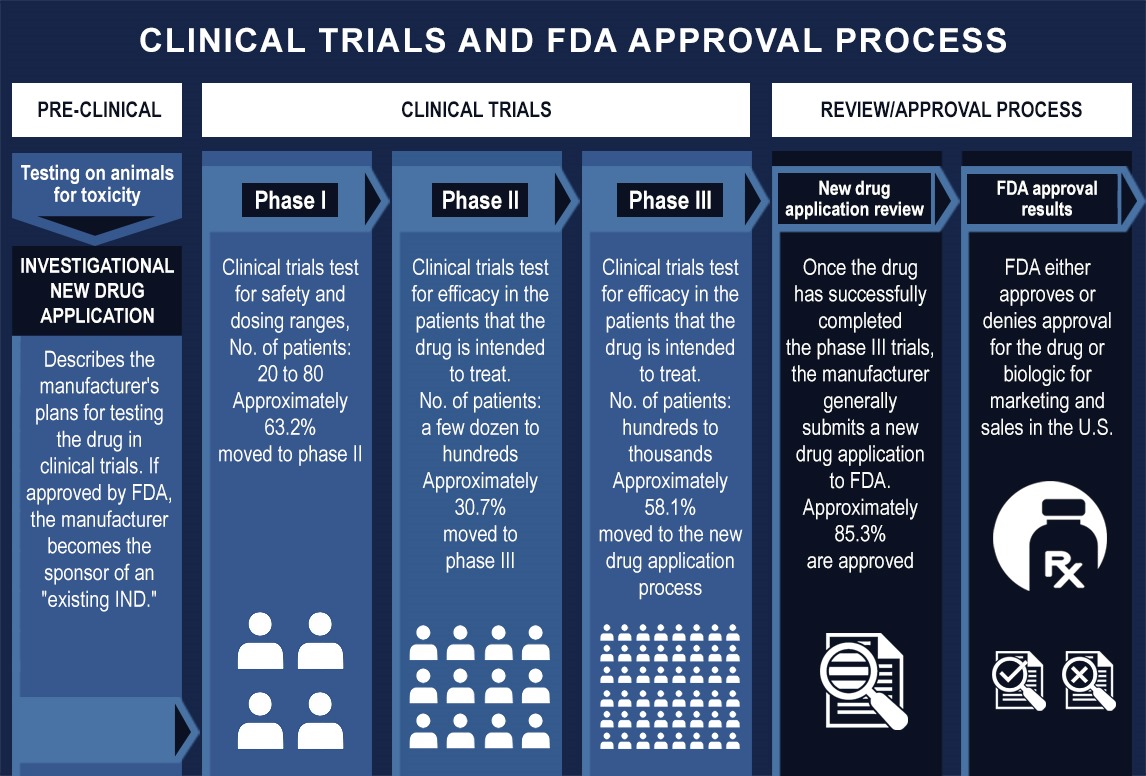
In the world of cell and gene therapy, growth is both the goal and the challenge. Early-stage biotech companies begin with a handful of samples, managed in spreadsheets and tracked manually. But what happens when your promising therapy enters Phase II, scales to multi-site trials, and eventually moves toward commercialization? The complexity of managing biological and cell therapy samples grows exponentially, and manual processes simply can’t keep up. This is where a scalable sample inventory system, implemented step by step becomes essential for managing biological and cell therapy samples. In this use case deep dive, we explore how the right system can support your sample operations from R&D through to global commercial readiness.
The Challenge: Manual Systems Don’t Scale
Manual sample tracking methods (like spreadsheets or homegrown databases) work in early R&D phases. But as trial complexity grows, these methods hit breaking points:
- Volume strain: Hundreds of samples become thousands across different trial arms.
- Multi-site complexity: Samples are now handled at multiple locations, each with its own procedures.
- Audit risk: Regulatory expectations increase with scale, and manual logs can’t provide complete traceability required for FDA audit readiness.
- Real-time visibility gaps: Trial managers need centralized insight, not siloed spreadsheets.
Scaling isn’t just about more samples, it’s about managing risk, speed, and accuracy as you grow.
Scaling Sample Management Through Each Phase
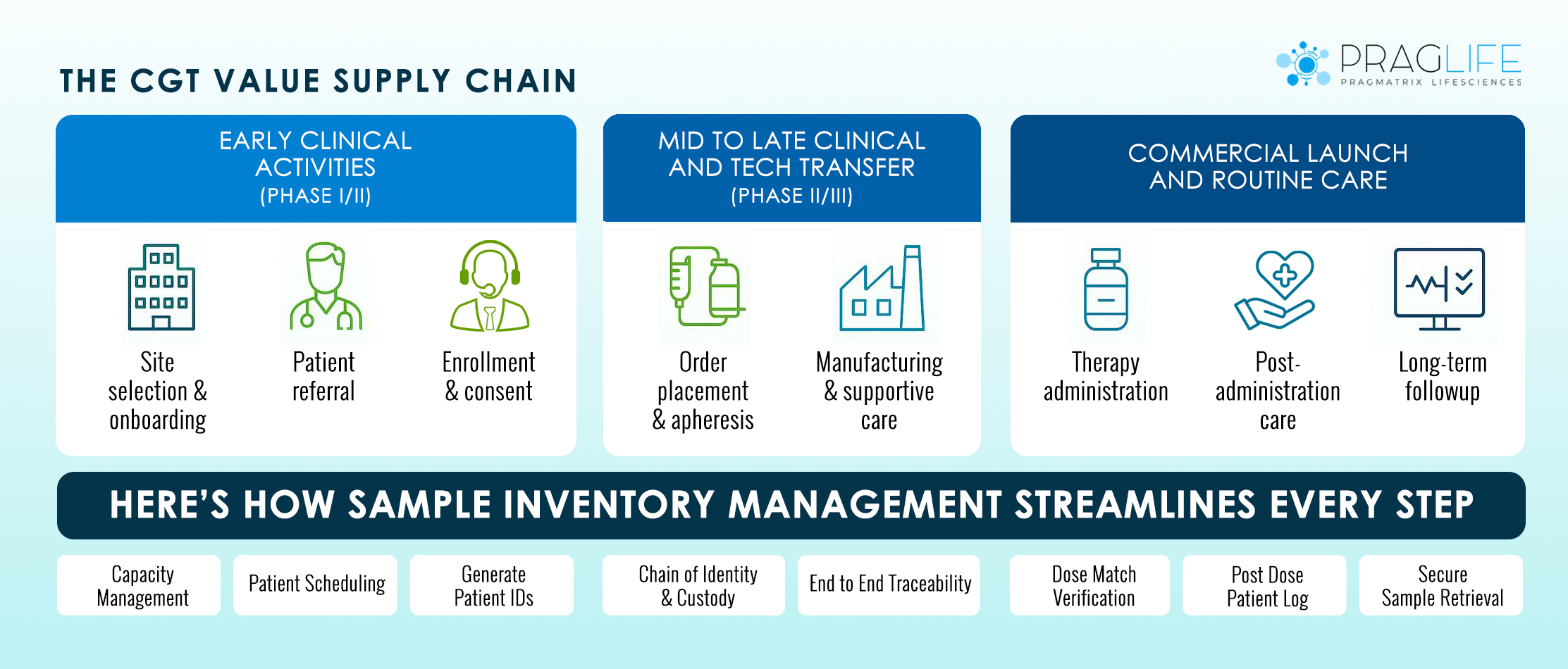
Let’s walk through how a modern storage inventory system supports biotech teams through real growth phases:
Phase I: R&D and Preclinical
- Pain Point: Manual tracking is still manageable, but errors already occur.
- Inventory System Advantage: Foundation-level implementation (freezer mapping, barcode integration) ensures clean setup.
Phase II: Clinical Trials Begin
- Pain Point: As sample volume grows, maintaining temperature logs and enforcing SOPs becomes essential for meeting regulatory compliance standards.
- Inventory System Advantage:
- Automated audit trails begin tracking all movements
- Real-time sample location and freezer availability
- Alerts for expirations or temperature excursions
Phase III: Late-Stage Clinical Trials
- Pain Point: Multi-site coordination increases complexity; compliance demands intensify.
- Inventory System Advantage:
- Centralized visibility across trial sites
- Role-based access controls and real-time data syncing
- Integration with LIMS or ELN systems
Commercial Scale-Up
- Pain Point: Inconsistent documentation, recall risk, and scaling infrastructure
- Inventory System Advantage:
- Full GMP compliance support (21 CFR Part 11, CGMP workflows)
- Configurable workflows for batch release and deviation management
- Analytics dashboards for inventory forecasting and space utilization
Why Scalability Isn’t Just a Feature, It’s a Requirement
As your organization grows, you’re not just scaling volume. You’re scaling:
- Compliance burden
- Documentation complexity
- Operational risk
- Stakeholder expectations
Without the right infrastructure, sample management becomes a bottleneck. A scalable inventory system doesn’t just help you cope with growth it enables it.
Real-World Impact
Biotech organizations that implement scalable sample inventory systems early report:
- 70% reduction in sample retrieval errors during clinical trials
- 60% faster readiness for regulatory audits
- 80% less time spent reconciling records during inspections
These aren’t just operational wins, they’re business enablers.
Conclusion: Build for the Future Now
Your sample management approach shouldn’t hold back your growth. Whether you’re just entering Phase I or planning global commercialization, a scalable inventory system is your operational backbone.
Looking to build a foundation that supports growth, quality, and compliance? Let’s talk about how PragLife’s Storage Inventory System can grow with you from day one to long-term success.




